Chemistry.
Content
There are two paths that you can take for learning chemistry content.
The more popular of the two is binge watching MSJ Chem on YouTube. That’s for all of you “visual learners.”
The other way, the one that I went with, is reading through Kognity’s course notes. It is an absolute god tier resource for chemistry content. You could honestly get away with only doing content from Kognity without any past paper practice and pulling a 7 in your final, though I wouldn’t recommend that for obvious reasons. It has everything you need and covers every single question that could possibly come. There is no additional, unnecessary, or “nice to know” information. It is all to the point and perfect. They really did a good job with their chemistry notes.
You would need to buy a subscription for it if you want to use it though, which I understand may drive some of you away. My school fees included a subscription to Kognity, but yours likely doesn’t, so MSJ Chem is absolutely perfect.
There are other alternative YouTube channels as well. Here are my top choices:
These are in order of best to worst (in my opinion). Only MSJ Chem is specifically for IB chemistry (the best), whereas the others just cover the topics in general.
If you want more help and resources, I recommend checking out IB Dead.
Paper 1 and 2
IB doens't tend to ask very complicated questions for Chemistry. As long as your really know your content, you really won't face any problems. However, there are some topics and some pieces of content that are tricky and complex. Below is some of the content I found quite challenging to understand/memorize.
1) Periodic Trends (Topic 3: Periodicity)
Periodic trends (source: University of Alabama SSC)
Preview PDFImportant exception to note (ionization energy):
Across a period include decreases from beryllium to boron, magnesium to aluminum, nitrogen to oxygen, and phosphorus to sulfur. These drops are due to electron configurations: p orbital electrons are higher in energy and further from the nucleus than s orbital electrons, making them easier to remove. Additionally, electrons in doubly occupied orbitals experience repulsion, requiring less energy to remove.
2) Enthalpy change formulae
Combustion (ΔHc)
ΔHc = ΣΔHf(reactants) - ΣΔHf(products)Formation (ΔHf)
ΔHf = ΣΔHf(products) - ΣΔHf(reactants)Using bond enthalpies (ΔHbond)
ΔHbond = ΣΔH(bonds broken) - ΣΔH(bonds formed)
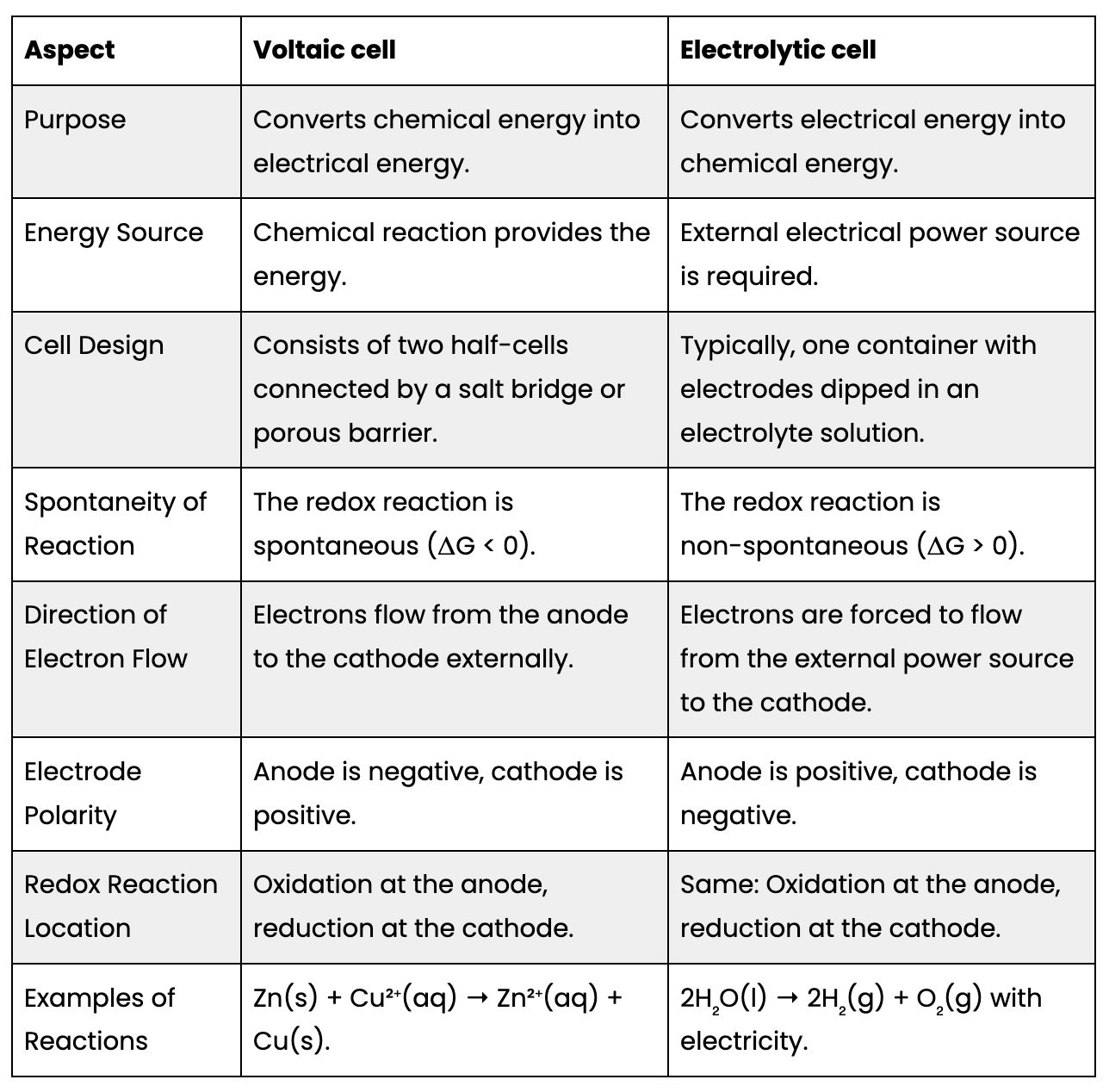
3) Voltaic vs Electrolytic cells
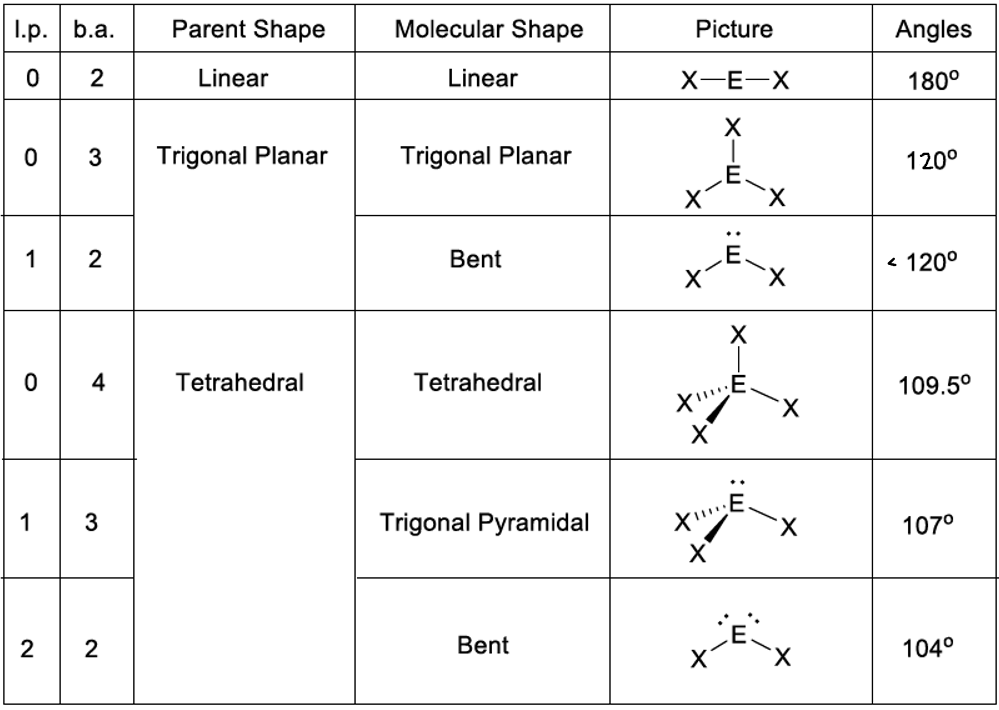
4) VSEPER theory (source: Wizeprep)
5) Organic chemistry reactions
Watch the video below for a brief overview on all reactions that you will need for organic chem.
Paper 3
Just know the content for paper 3 as well. You don't really need to worrry about the experimental questions, just focus on your option. Use MSJ chem videos and you'll be just fine!